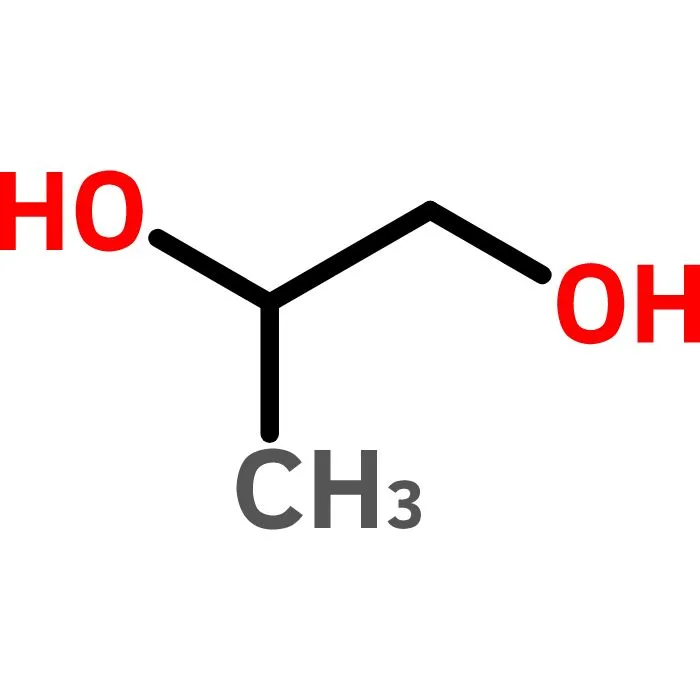
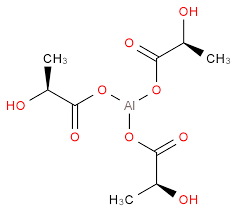
Minimum assay (G.C.): 99.5%
Identity according to Pharmacopoeias:: passes test
Density 25/25: 1.035-1.037
Density 20/20: 1.035-1.040
Range of Distillation (min. 95%): 184-189°C
Refractive Index n 20/D: 1.431-1.433
Maximum limit of impurities
Appearance: passes test
Acidity (Ph. Eur.): passes test
Acidity (USP, JP): passes test
Reducing substances: passes test
Residue on ignition (as SO4): 0.005 %
Chloride (Cl): 0.007%
Sulfate (SO4): 0.002%
Oxidizing substances: passes test
Residual solvents (Ph.Eur/USP): passes test
Glycerol: passes test
Ethylenglycol: 0.10%
Diethyleneglycol: 0.10%
Water (H2O): 0.1 %







 Български
Български Back
Back